is the study of the of the composition, structure and properties of matter.
An ionized gas is in the plasma state of matter.
In plasma, the gas is energized to the point where its atoms or molecules lose electrons, creating a mixture of positively charged ions and free electrons. This ionization occurs at high temperatures or under strong electromagnetic fields.
Because plasma is made up of charged particles, it has unique properties compared to the other states of matter (solid, liquid, and gas). Plasmas can conduct electricity, generate magnetic fields, and respond to electric and magnetic forces.
At ultracold temperatures near absolute zero (0 K or -273.15°C), matter behaves in some extraordinary and counterintuitive ways due to the dominance of quantum effects. Here’s what happens when temperatures approach this extreme:
- Bose-Einstein Condensates are a state of matter that forms when a group of bosons (particles with integer spin, such as certain atoms or photons) are cooled to temperatures close to absolute zero.
- At these temperatures, the individual atoms in a gas slow down and merge into a single quantum state, behaving like a macroscopic wave rather than discrete particles. All the atoms move in the same way, as if they are a single entity.
- BECs exhibit quantum mechanical effects on a macroscopic scale, such as interference patterns (where waves interact) and superfluidity (flowing without friction).
- The first BEC was created in 1995 by cooling a gas of rubidium atoms to just a few billionths of a degree above absolute zero.
A homogeneous mixture is a mixture in which the components are uniformly distributed throughout and you cannot easily distinguish the individual substances. Here are some common examples:
- A solution of salt dissolved in water is a homogeneous mixture because the salt is evenly distributed throughout the water. Once dissolved, the individual salt particles are not visible, and the solution has a uniform composition.
- The air we breathe is a homogeneous mixture of gases like oxygen, nitrogen, carbon dioxide, and trace amounts of other gases. These gases are evenly mixed and form a uniform composition.
- When sugar is dissolved in tea or coffee, it forms a homogeneous mixture. The sugar molecules disperse evenly throughout the liquid, and the solution appears uniform.
- Brass (a mixture of copper and zinc) or stainless steel (a mixture of iron, chromium, and other metals) are homogeneous at the atomic level. These alloys have a uniform composition throughout, even though they consist of different metals.
- Vinegar is a homogeneous mixture of acetic acid and water. The acetic acid molecules are evenly distributed in the water, so you cannot distinguish them apart.
- Many commercial air fresheners are homogeneous mixtures where fragrance oils are dissolved in a solvent (usually water or alcohol). The fragrance is evenly dispersed in the solution.
- Wine is a homogeneous mixture of water, alcohol (ethanol), sugars, and other compounds. The components are thoroughly mixed and uniformly distributed.
- Homogenized milk is a uniform mixture of fat globules and water. The fat droplets are broken down and evenly distributed throughout the liquid, making the milk appear uniform.
-
A mixture that has a non-uniform composition is known as a heterogeneous mixture.
- Salad: A salad with lettuce, tomatoes, cucumbers, and dressing has different visible components and doesn’t have a uniform composition.
- Sand and Water: Sand mixed with water is a heterogeneous mixture because the sand particles don’t dissolve in the water and remain separate.
- Oil and Water: When oil and water are mixed, they form a heterogeneous mixture, where the oil floats on top of the water because they don’t mix uniformly.
- Granite: Granite is a rock that consists of different minerals like quartz, feldspar, and mica, which can be seen with the naked eye.
- Concrete: Concrete is a mixture of cement, water, sand, and gravel, where the individual components are not uniformly distributed.
Physical properties are characteristics of a substance that can be observed or measured without changing the substance’s chemical identity. In other words, physical properties describe the appearance, texture, or behavior of a material without altering its molecular structure.
A chemical change (or chemical reaction) occurs when a substance is transformed into a different substance with a new chemical composition.
- Burning of Wood
- Rusting of Iron
- Baking a Cake
- Digestion of Food
- Reaction of Vinegar and Baking Soda
The property of matter that depends on the size of the sample is called an extensive property.
Extensive properties are those that change when the amount of the substance changes. In other words, the value of these properties depends on the size or quantity of the sample. The more material you have, the greater the value of the extensive property.
PPM stands for “Parts Per Million”.
It is a unit of concentration commonly used to measure very dilute concentrations of substances in a solution or mixture. It expresses the amount of one substance relative to a million parts of the total mixture or solution. In simple terms, 1 PPM means 1 part of a substance per 1 million parts of the total.
The unit commonly used to measure power is the watt (symbol: W), which is a derived unit of power in the International System of Units (SI).
1 watt (W) is equal to:
- 1 joule per second (J/s).
In other words, if 1 joule of energy is used in 1 second, the power is 1 watt.
Dimensional analysis is a mathematical technique used to understand the relationships between physical quantities by analyzing their dimensions (i.e., the fundamental units they are expressed in, such as length, mass, time, etc.). A method for solving problems using unit conversions.
A physical change is a change in which the appearance or state of a substance changes, but its chemical composition remains the same.
- Melting of Ice:
- Boiling Water:
- Breaking a Glass:
- Dissolving Salt in Water:
- Tearing Paper:
- Chopping Wood:
- Crushing a Can:
- Sublimation of Dry Ice:
The unit joule (J) is the SI unit of energy, and it is used to measure various types of energy in physics and other fields.
The term that refers to the ratio of distance traveled to time taken is speed.
- Speed is a scalar quantity (it has magnitude but no direction) and is defined as the distance traveled per unit of time.
- The formula for speed is: Speed=Distance/Time
- Speed=100km/2H= 50KM/H
A SINGLE TYPE OF PARTICLE
An intensive property is a physical property of a substance that does not depend on the amount or size of the material present.
Examples of Intensive Properties:
-
Density:
- Density is the mass per unit volume of a substance and is independent of the amount of substance. For example, the density of water is 1 g/cm³, regardless of whether you have a glass of water or a whole lake of water.
-
Temperature:
- The temperature of a substance is an intensive property because it does not change with the amount of material. A cup of water at 50°C will have the same temperature as a gallon of water at 50°C.
-
Boiling Point:
- The boiling point is the temperature at which a substance changes from a liquid to a gas. For example, the boiling point of pure water is 100°C at standard atmospheric pressure, regardless of how much water you have.
-
Melting Point:
- The melting point is the temperature at which a solid turns into a liquid. For example, the melting point of ice is always 0°C, regardless of whether you have a small piece of ice or a large iceberg.
-
Refractive Index:
- The refractive index is a measure of how much light bends when it enters a material. For example, the refractive index of water is around 1.33, and this value doesn’t change based on the quantity of water.
-
Color:
- The color of a substance is an intensive property because it is independent of the amount of material. A red liquid will be red whether you have a drop of it or a liter of it.
-
Hardness:
- Hardness refers to the resistance of a material to deformation (such as scratching). For example, diamonds are hard regardless of the size of the diamond.
-
Electric Conductivity:
- The ability of a substance to conduct electricity is an intensive property. Whether you have a small wire or a large one, the conductivity of the material remains constant as long as the temperature and other conditions are the same.
-
Pressure:
- Pressure in a system, if uniform, is also an intensive property. It does not depend on the size of the system, though the total force would.
The unit for measuring volume in the International System of Units (SI) is the cubic meter (m³).
When performing addition or subtraction with significant figures, the rule is based on decimal places, not the number of significant digits.
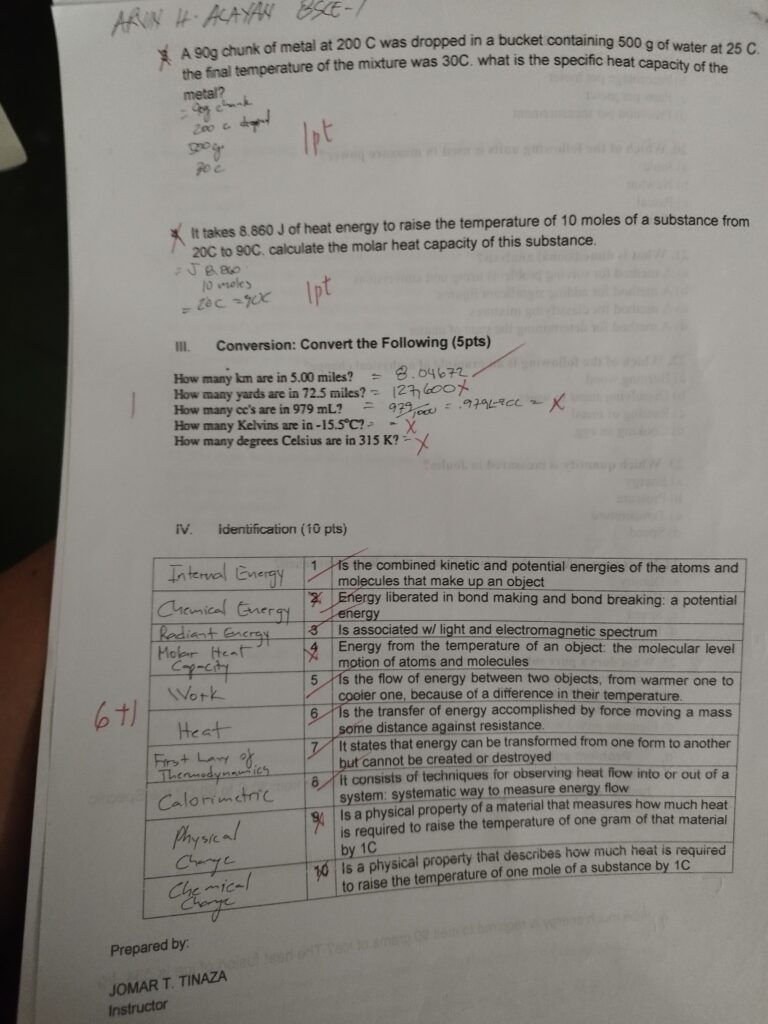
The combined kinetic and potential energies of the atoms and molecules that make up an object is referred to as its internal energy (often symbolized as UUU)
Chemical energy is the energy stored in the bonds of chemical compounds (like atoms and molecules). It is a form of potential energy, as it has the potential to do work during a chemical reaction when bonds are either formed or broken.
RADIANT ENERGY





Gave 666zk a try and I wasn’t disappointed! The games were pretty entertaining, and I liked the overall design. Worth a look! Check it out at 666zk.
The Y9Betapp is actually pretty decent! The mobile app is smooth and easy to use, which is a big plus for me since I’m always on the go. They could improve the live betting interface, but overall a good experience. Download y9betapp today!
Downloaded the kim88app. It’s okay and very smooth. It is very colorful and vibrant, check it out ! It is good for killing time. kim88app
Heard about 777pubcc from my friends. Gonna try my luck tonight! Wish me luck, guys! More info here: 777pubcc